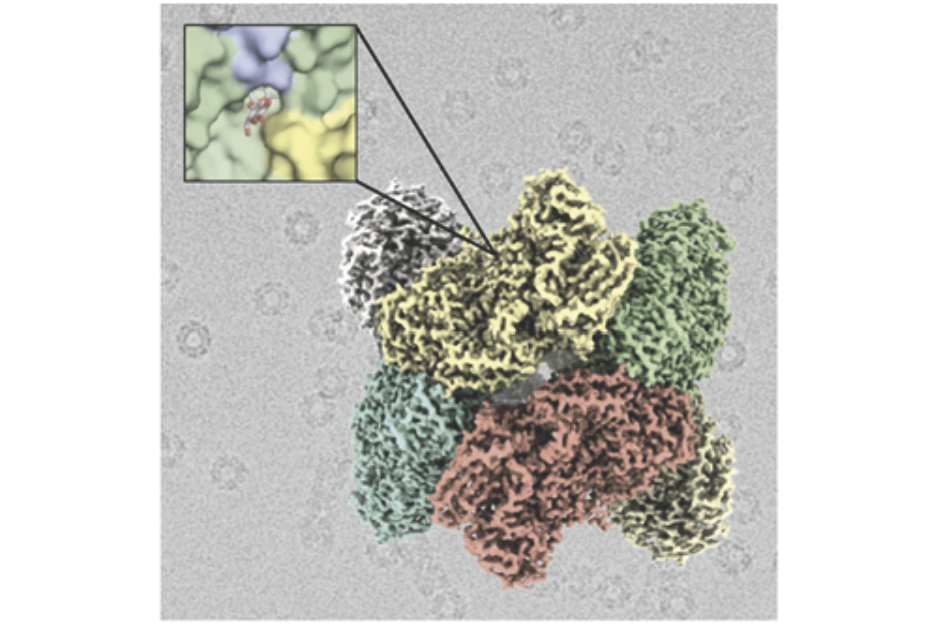
- Researchers from the CNIO and the CSIC have elucidated by means of electronic cryomicroscopy the molecular structure of a thermoresistant enzyme, which has the property of hydrolyzing lactose
-
The finding, which represents a significant advance in the development of new procedures for obtaining lactose-free milk and milk products, was published in the journal ACS Chemical Biology
Researchers from the Centro Nacional de Investigaciones Oncológicas (CNIO), the Instituto de Química Física Rocasolano (IQFR) of the Consejo Superior de Investigaciones Científicas (CSIC) and the Instituto de Agroquímica y Tecnología de Alimentos (IATA, CSIC), have elucidated by means of electronic cryomicroscopy the molecular structure of an ß-galactosidase enzyme, which had not been able to be resolved by conventional techniques. This enzyme has the property of hydrolyzing lactose and is being used in the development of domestic devices to convert normal milk into lactose-free milk. The results of the work, which has been co-lead by Rafael Fernández-Leiro, from CNIO, and Julia Sanz-Aparicio, from IQFR-CSIC, are published in the journal ACS Chemical Biology.
"Lactose intolerance is a common digestive disorder that affects a large proportion of the adult human population. The severity of symptoms varies from person to person, and depends on the susceptibility to sugar and the amount ingested. For this reason, in the field of biotechnology, the study of enzymes that can be used for the production of lactose-free milk and milk derivatives, such as β-galactosidase from the bacterium Thermotoga maritima or TmLac, has acquired great importance", explains Julio Polaina, a CSIC researcher at IATA.
Rafael Fernández-Leiro adds that "we have used the technique of electronic cryomicroscopy or Cryo EM, which has become an alternative to the X-ray diffraction traditionally used to resolve the structure of macromolecules at high resolution. The structure of the TmLac enzyme had been attempted previously without success. Thanks to Cryo EM we have been able to resolve its structure with a resolution of two angstroms, one of the highest resolutions achieved to date using this technique and which allows us to see the atomic structure of this enzyme in detail".
"Our innovation makes an important contribution to the health and well-being of the large population with varying degrees of lactose intolerance. A next step is the design of hybrid enzymes that can be efficiently bound to different solid supports and thus be used for different applications", concludes Julia Sanz-Aparicio.
Samuel Míguez Amil, Elena Jiménez-Ortega, Mercedes Ramírez-Escudero, David Talens-Perales, Julia Marín-Navarro, Julio Polaina, Julia Sanz-Aparicio y Rafael Fernández-Leiro. The cryo-EM Structure of Thermotoga maritima β‑Galactosidase: Quaternary Structure Guides Protein Engineering. ACS Chem. Biol. DOI: 10.1021/acschembio.9b00752
Image: Structure of the enzyme ß-galactosidase obtained by electron cryomicroscopy.

